This website uses a variety of cookies, which you consent to if you continue to use this site. You can read our Privacy Policy for
details about how these cookies are used, and to grant or withdraw your consent for certain types of cookies.
Corrosion and Galvanized Steel Flumes & Weirs
 Galvanized steel is commonly used for flumes and (somewhat less so) weirs in irrigation and water rights applications. Galvanized steel is economical and can provide a good useful lifespan.
Galvanized steel is commonly used for flumes and (somewhat less so) weirs in irrigation and water rights applications. Galvanized steel is economical and can provide a good useful lifespan.
However, unlike fiberglass or stainless steel which are generally predictable in how long they will last, galvanized flumes / weirs in freshwater applications can experience dramatically different lifespans due to relatively small differences in water content or conditions.
Differing Lifespans
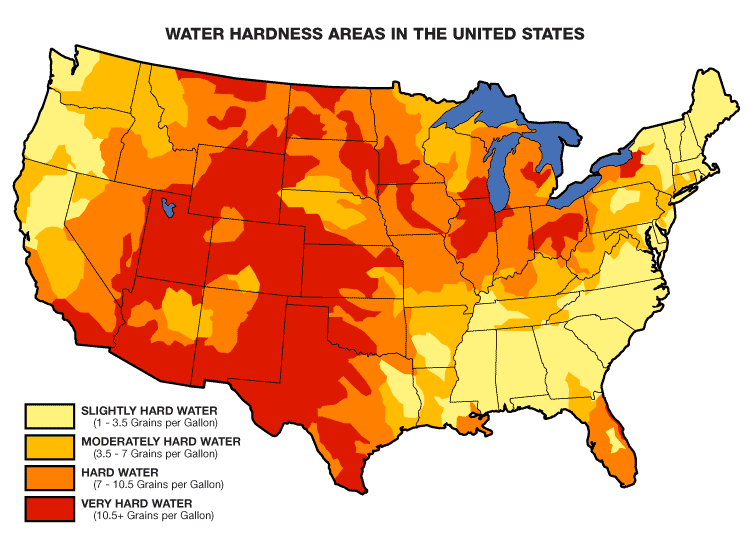
The rate of corrosion of zinc in fresh water is generally much less in hard water than in soft water. For installations in the Rocky Mountains, this can mean shorter useful lives than ones in central / northern California (the two areas of greatest use of flumes for water rights apportionment).
Factors Affecting Corrosion
Dissolved gasses, hardness (mineral rate), flow rate, and ions/chlorides are all contributing factors to the rate of zinc corrosion.
Gasses:
- Oxygen creates corrosion. The more oxygen in the water, the greater the rate of corrosion of zinc. Boundary zones where air and water meet have greater dissolved oxygen concentrations corrode more quickly than those submerged.
- For flumes: waterlines and discharge sections see the greatest corrosion.
- For weirs: waterlines, weir crests, and downstream weir pools see the greatest corrosion.
Hardness:
- In hard water, zinc combines with carbonates and bio carbonates to form zinc carbonate, which unlike zinc oxide, is not water soluble.
- The zinc carbonate deposits on the surface of the zinc and creates a passive film on the galvanized part slowing corrosion.
- The softer the water, the lower it is in carbonate; therefore, soft water is more corrosive than hard.
Flow Rate:
- The greater the flow rate, the greater the rate of corrosion as the friction of the water increases abrasion of the wetted surfaces of flumes and weirs.
Other Ions:
- Choloride is the most aggressive ion to zinc and tends to be more pronounced in soft waters than hard waters.
- The protective film deposited by carbonate in hard water protects the zinc coating from anion attack.
Extending the Lifespan of Galvanized Flumes / Weirs
To extend the life of galvanized flumes and weirs they should be periodically inspected. If corrosion is found it should be immediately cleaned off and cold galvanization applied.
Keep in mind that cold galvanization does not typically last as long as hot dipped galvanization – so treated areas will most likely need periodic retreatment.
Image: H2O Distributors
Source: American Galvanizers Association
Related Blog Posts
Explore more insights in our blog.
LOCATIONS IN ATLANTA, GA & BOISE, ID




